The role of glucose transporter type 1 (GLUT1) and carbonic anhydrase IX (CAIX) expression by prostate adenocarcinoma tissue in determining disease prognosis and effectiveness of radical treatment
https://doi.org/10.21886/2308-6424-2022-10-4-13-20
Abstract
Introduction. Today, due to the insufficient diagnostic accuracy of existing tools for determining clinically significant forms of prostate cancer, the search for new indicators that predict the course of the disease and the effectiveness of radical treatment is relevant. Various malignant tumors could increase glucose consumption and grow under hypoxic conditions. It seems promising to assess the expression level of glucose transporter type 1 (GLUT1) and carbonic anhydrase IX (CAIX) in prostate adenocarcinoma cells of different malignancy score.
Objective. To determine CAIX and GLUT1 expression in ISUP grades 1-5 prostate adenocarcinoma cells for evaluation of the disease prognosis and radical prostatectomy effectiveness.
Materials and methods. Immunohistochemical study of postoperative material after radical prostatectomy with determination of GLUT1 and CAIX expression by tumor cells was carried out. The presence or absence of biochemical recurrence within one year after surgery was determined. The correlation between the level of expression, the presence of biochemical relapse and a few other clinical parameters was determined.
Results. GLUT1 expression level statistically significant correlated with ISUP 4 and 5 (r = 0.457, p < 0.0001), prostate-specific antigen (PSA) level (r = 0.378, p < 0.0001), pT3b disease stage (r = 0.380, p < 0.0001), extracapsular extension (r = 0.355, p = 0.001), and inversely correlated with ISUP 1 (r = -0.274, p = 0.009). CAIX immunoexpression was observed in 10.0% of samples and the intensity was low (< 20% of cells).
Conclusion. Elevated expression of glucose transporter type 1 (GLUT1) by prostate adenocarcinoma cells among patients after radical prostatectomy is associated with high grade of malignancy (ISUP 4 and 5), pT3b disease stage, extracapsular extension of the tumor, as well as high PSA, which allows using it for the prognosis evaluation.
For citations:
Vovdenko S.V., Morozov A.O., Avraamova S.T., Alexandrov N.S., Zharkov N.V., Kozlov V.V., Kogan E.A., Bezrukov E.A. The role of glucose transporter type 1 (GLUT1) and carbonic anhydrase IX (CAIX) expression by prostate adenocarcinoma tissue in determining disease prognosis and effectiveness of radical treatment. Urology Herald. 2022;10(4):13-20. (In Russ.) https://doi.org/10.21886/2308-6424-2022-10-4-13-20
Introduction
Currently, the prognosis of the course of prostate cancer is determined by such indicators as the level of prostate-specific antigen (PSA), disease stage, and degree of tumor cells differentiation at a morphological examination. However, despite an extensive arsenal of modern diagnostic methods, a significant number of cases of both overdiagnosis and treatment of clinically insignificant forms of the disease and late detection of highly malignant and prognostically unfavorable forms of prostate cancer persist in the world, which indicates the need to search for additional markers to assess the disease prognosis and treatment effectiveness [1][2].
Increased intensity of glucose uptake compared to healthy tissues, as well as active growth and development under hypoxic conditions, are typical for many malignant tumors. Prostate cancer is not an exception. There is data on the association between a high level of hypoxia in tumor tissue with castration refractoriness development and a decrease in hormonal therapy efficacy in prostatic adenocarcinoma [3], and between an increased level of glucose consumption by tumor tissues due to a high level of glucose transporter type 1 (GLUT1) expression with active tumor metastasis, lymph nodes affection, tumor volume, and disease progression [4]. Thus, evaluation of the expression of such markers as CAIX, responsible for maintaining normal pH in the cell under hypoxia, and GLUT1, responsible for glucose transport across the cytoplasmic cell membrane, can be a promising direction to determine the disease prognosis.
In the present study, the authors evaluated the level of CAIX and GLUT1 expression in prostatic adenocarcinoma tissue of different degrees of malignancy and further determined its role in predicting the course of the disease and the oncological efficacy of the radical prostatectomy performed.
The study aimed to determine CAIX and GLUT1 expression in ISUP grades 1 – 5 prostate adenocarcinoma cells for evaluation of disease prognosis and radical prostatectomy effectiveness.
Materials and Methods
From June 2015 to March 2017, the authors analyzed 100 samples of postoperative specimens of patients after radical prostatectomy for prostate cancer performed at the clinic of the Institute of Urology and Human Reproductive Health, the Sechenov University in patients with morphologically confirmed adenocarcinoma of the prostate. The analysis was carried out based on the Centralized Pathology Department, the Sechenov University. The research team estimated parameters such as age, prostate volume, the International Society of Urological Pathology (ISUP) score (the Gleason score was determined according to the scale modified by Epstein et al. (2016)), the level of PSA, the presence of biochemical recurrence (PSA increase > 0.2 ng/ml) within 2 years, tumor spread beyond the gland capsule, and the presence of a positive surgical margin. Clinical data are shown in Table.
Table. Characteristics of patients and specimens
|
Attribute |
n (%) |
|
Age, yrs |
|
|
< 65 |
58 |
|
> 65 |
42 |
|
pT |
|
|
pT2 |
66 |
|
pT3a |
7 |
|
pT3b |
25 |
|
pT4 |
2 |
|
PSA level, ng/ml |
|
|
< 10 |
47 |
|
> 10 |
53 |
|
Gleason score (ISUP grade) |
|
|
3 + 3 (ISUP1) |
20 |
|
3 + 4 (ISUP2) |
20 |
|
4 + 3 (ISUP3) |
20 |
|
4 + 4 (ISUP4) |
20 |
|
≥ 9 (ISUP5) |
20 |
|
Biochemical recurrence |
|
|
Yes |
19 |
|
No |
81 |
|
Perineural invasion |
|
|
Yes |
17 |
|
No |
83 |
|
Extraprostatic extension |
|
|
Yes |
14 |
|
No |
86 |
The immunohistochemical study included antibodies such as rabbit monoclonal antibody GLUT1, AC-0132A (“Epitomics, Inc.”, Burlingame, CA, USA), rabbit monoclonal antibody Carbonic Anhydrase IX (CAIX), 379R-14 RUO Cell Marque™ (“Sigma Aldrich Corp.”, Saint-Lewis, MO, USA).
The study was performed according to the standard protocol. The tissue was fixed in buffered formalin with subsequent waxing of the sample. Sections 5 μm thick were placed on poly-L-lysine glasses, dewaxed, and rehydrated. Endogenous peroxidase was blocked with a 3% hydrogen peroxide solution for 10 minutes. Then, 50 μl of diluted primary serum was applied to the sections for the immunohistochemical reaction, followed by incubation for 30 minutes at 37 °C. When satisfactory staining was achieved, the sections were washed with distilled water and stained with Meyer hematoxylin for 3 minutes.
The intensity of GLUT1 and CAIX expression was assessed in the tumor epithelium by two pathologists independently. If there was a discrepancy in the assessment, a third invited specialist formed the final decision. Expression intensity was expressed in points, where 0 was no expression, 1 was expression in < 20% of cells, 2 was expression in 20% – 40% of cells, and 3 was expression in > 40% of cells.
Statistical analysis. The data were processed using the IBM SPSS Statistics 20.0 software (“SPSS: An IBM Company”, “IBM SPSS Corp.”, Armonk, NY, USA). The data were tested for normality of distribution using the Shapiro-Wilk and Kolmogorov-Smirnov tests. The level of p < 0.05 at a = 0.05 was considered statistically significant.
Results
The immunohistochemical assay revealed the expression of CAIX and GLUT1 markers, which manifested as staining of the cytoplasmic membrane of tumor cells. GLUT1 immunoexpression was absent in only one tumor specimen. There was expression in < 20.0% of cells in 58.0% of samples and expression in 20.0% – 40.0% of cells in 40.0% of samples. The Spearman’s correlation showed that the degree of GLUT1 expression was significantly directly correlated with the degree of ISUP 4 and 5 malignancy (r = 0.457, p < 0.0001) (Fig. 1B), the PSA level (r = 0.378, p < 0.0001), the disease stage pT3b (r = 0.380, p < 0.0001), extracapsular tumor spread (r = 0.355, p = 0.001), and correlated inversely with ISUP1 malignancy (r = -0.274, p = 0.009) (Fig. 1A) and the disease stage pT2c (r = -0.423, p < 0.0001). CAIX immunoexpression was observed in only 10.0% of samples and was of low severity (< 20.0% of cells). The only statistically significant correlation concerning CAIX was between its expression in tumor cells and the expression level of GLUT1 (r = 0.331, p = 0.01) (Fig. 2).
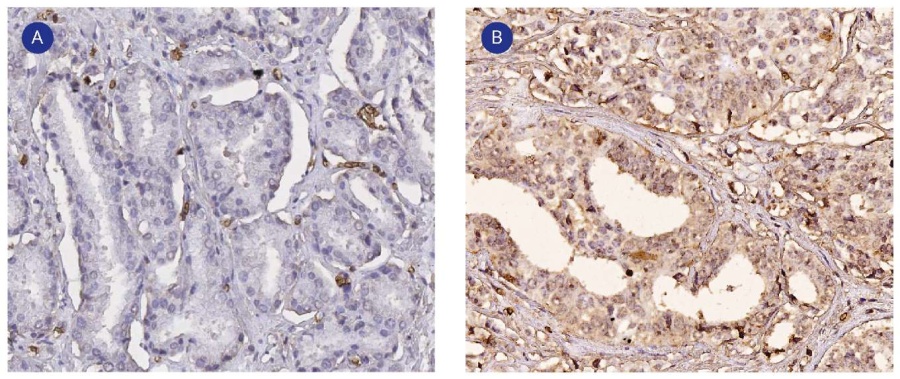
Figure 1. GLUT1 expression by ISUP 1 (A) and ISUP 4-5 (B) prostatic adenocarcinoma cells. Immunohistochemical staining, magn. ×200
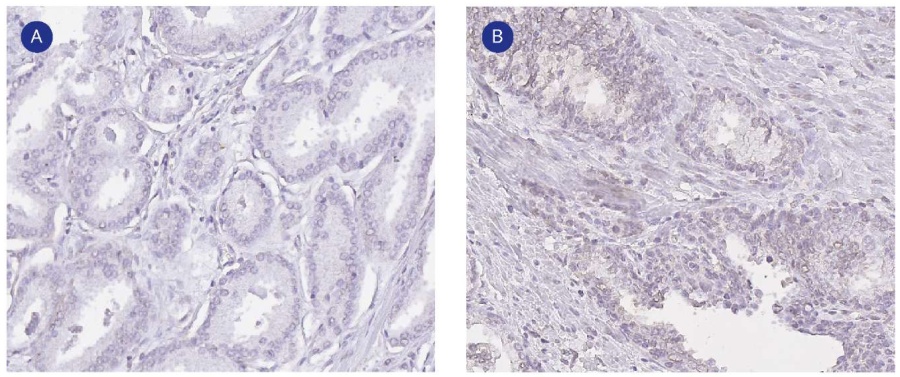
Figure 2. Immunohistochemical reaction of prostatic adenocarcinoma with CAIX antibodies: A — negative reaction of prostatic adenocarcinoma cells with CAIX antibodies (ISUP 3), magn. ×200; B — slightly positive CAIX expression by prostatic adenocarcinoma cells (ISUP 4 and 5), magn. ×100
Discussion
The peculiarity of the metabolism of healthy prostate cells is in an unusual way of glucose metabolism – aerobic glycolysis. Despite a sufficient supply of oxygen to the cells, the tricarboxylic acid cycle in the cell does not proceed completely, providing the cell with a smaller number of ATP molecules than in oxidative phosphorylation. This occurs because of the inhibition of the enzyme m-aconitase, which interacts with citrate, caused by a tenfold increase in the number of zinc ions in the cell cytoplasm. Citrate accumulated in a cell is further excreted into the intercellular matrix and is a part of prostate secretion [5][6]. In prostatic adenocarcinoma cells in the early stages of the disease, the main way of energy metabolism is oxidative phosphorylation. This ability for oxidative phosphorylation is acquired by cells by fully restoring the tricarboxylic acid cycle [7]. This occurs because of a decrease in the activity of zinc transporters, leading to a drop in its intracellular concentration [8][9].
It should be noted that in the early disease stages, there is a relatively low level of glucose consumption by malignant cells. However, as the degree of malignancy by Gleason score increases and hormonal refractoriness develops, the glucose uptake by tumor tissue also increases [10]. Thus, it can be assumed that the determination of glucose uptake rate by prostatic adenocarcinoma cells can serve as a diagnostic marker of disease prognosis. The family of glucose transporters (GLUTs) provides energy-independent transport of glucose and other hexoses across the cell cytoplasmic membrane per the accumulation gradient. Currently, 13 members of the family are known to have different affinities to hexoses, with GLUT1, GLUT2, GLUT3, and GLUT4 types being the most glucose-sensitive [11]. Of all types of glucose transporters in prostate tissue, GLUT1 has received the most attention. The results of studies concerning this transporter are heterogeneous and often contradict each other. One of the first studies on the expression of glucose transporters in hyperplasia and prostate cancer cells during an immunohistochemical study of hyperplasia and adenocarcinoma prostate tissue showed that hyperplastic prostate tissue expressed only GLUT1, while adenocarcinoma cells expressed GLUT1 and GLUT12 [12]. Another study by Reinicke et al. (2012) aimed to compare the expression of this marker in healthy prostate tissue, hyperplastic tissue, intraductal prostate cancer, prostatic intraepithelial neoplasia, and prostate adenocarcinoma and revealed that GLUT1 was only expressed in the first three [13]. Subsequent studies showed increased GLUT1 expression in both laboratory prostatic adenocarcinoma cell lines (LNCaP, LNCaP-R, PC-3, DU-145) and in postoperative material from hormone-sensitive and castration-refractory prostate cancer [14–19]. High expression was correlated with the Gleason score >7 and PSA >20 ng/mL [14], as well as among patients after radical prostatectomy, worse survival without biochemical recurrence, worse survival without castration-refractory prostate cancer, and worse survival without metastasis [4].
A decrease in pH within tumor cells associated with active metabolic processes is observed in the tissue of prostatic adenocarcinoma under conditions of insufficient oxygen supply, which can negatively affect their viability, leading to apoptosis. There are mechanisms that allow cells, including tumor cells, to survive in an acidic, low-pH environment, one of which is the carbonic anhydrase family. Carbonic anhydrases are a group of zinc-containing metalloenzymes involved in pH regulation and reversible conversion of carbon dioxide to bicarbonate and protons [20]. In response to hypoxia, they ensure cell viability by maintaining an optimal intracellular pH level (6.9 – 7.0) [21]. The first published study evaluating CAIX expression in prostatic hyperplasia and prostate cancer tissue (with Gleason grade 3–5) revealed the lack of significant immunoexpression in all tissue samples. The authors suggested that, despite hypoxia zones in prostate cancer tissues, the absence of CAIX expression was associated with other mechanisms of pH regulation in malignant cells [22]. Donato et al. (2011) in their study on the evaluation of CAIX expression levels in urogenital tumors and adrenal neoplasms, along with high expression levels in neoplasms containing light cells (renal cell carcinoma, urothelial carcinoma, Sertoli cell tumor, light cell adenocarcinoma, and cortical carcinoma), showed that the immunoexpression by healthy cells, prostatic adenocarcinoma cells, and small cell prostatic carcinoma cells was completely absent [23]. However, the work by Fiaschi et al. (2013) revealed CAIX expression both by prostate cancer cells of different laboratory lines (LNCaP, DU145, PC-3) and by stromal tumor cells called cancer-associated fibroblasts (CAFs) [24]. Similarly, in the study by Ambrosio et al. (2016), cytoplasmic and nuclear expression of CAIX was observed in laboratory prostate cancer lines LNCaP and PC-3, as well as in prostate biopsy specimens containing tumor cells. The expression was associated with the presence of the tissue-induced hypoxia factor HIF1α, indicating that CAIX may be a marker of tissue hypoxia. The expression level correlated positively with the stage and degree of malignancy of prostate cancer [25]. At the same time, target inhibition of CAIX in prostate cancer tissue led to a decrease in pH in cells under hypoxic conditions, which caused a decrease in cell growth rate and an increase in apoptosis intensity [26].
The data obtained in the current study regarding GLUT1 expression in tumor cells correlate with the available publications and show its potential use as a marker for determining the prognosis of the disease. A significant correlation between CAIX expression and significant clinical parameters was not revealed, which may indicate the presence of other mechanisms of pH regulation in prostatic adenocarcinoma cells.
Conclusion
Elevated immunoexpression of the glucose transporter type 1 (GLUT1) by prostate adenocarcinoma cells after radical prostatectomy is associated with a high degree of malignancy (ISUP 4 and 5), disease stage, morphologically confirmed extracapsular spread of the tumor, and high PSA values, which allows specialists to use this marker in the evaluation of the disease prognosis.
References
↑1. Loeb S, Bjurlin MA, Nicholson J, Tammela TL, Penson DF, Carter HB, Carroll P, Etzioni R. Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014;65(6):1046-55. https://doi.org/10.1016/j.eururo.2013.12.062
↑2. Catalona WJ. Prostate Cancer Screening. Med Clin North Am. 2018;102(2):199-214. https://doi.org/10.1016/j.mcna.2017.11.001
↑3. Stewart GD, Ross JA, McLaren DB, Parker CC, Habib FK, Riddick AC. The relevance of a hypoxic tumour microenvironment in prostate cancer. BJU Int. 2010;105(1):8-13. https://doi.org/10.1111/j.1464-410X.2009.08921.x
↑4. Meziou S, Ringuette Goulet C, Hovington H, Lefebvre V, Lavallée É, Bergeron M, Brisson H, Champagne A, Neveu B, Lacombe D, Beauregard JM, Buteau FA, Riopel J, Pouliot F. GLUT1 expression in high-risk prostate cancer: correlation with 18F-FDG-PET/CT and clinical outcome. Prostate Cancer Prostatic Dis. 2020;23(3):441-448. https://doi.org/10.1038/s41391-020-0202-x
↑5. Costello LC, Feng P, Milon B, Tan M, Franklin RB. Role of zinc in the pathogenesis and treatment of prostate cancer: critical issues to resolve. Prostate Cancer Prostatic Dis. 2004;7(2):111-7. https://doi.org/10.1038/sj.pcan.4500712
↑6. Costello LC, Franklin RB, Feng P. Mitochondrial function, zinc, and intermediary metabolism relationships in normal prostate and prostate cancer. Mitochondrion. 2005;5(3):143-53. https://doi.org/10.1016/j.mito.2005.02.001
↑7. Pértega-Gomes N, Baltazar F. Lactate transporters in the context of prostate cancer metabolism: what do we know? Int J Mol Sci. 2014;15(10):18333-48. https://doi.org/10.3390/ijms151018333
↑8. Franklin RB, Costello LC. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch Biochem Biophys. 2007;463(2):211-7. https://doi.org/10.1016/j.abb.2007.02.033
↑9. Franz MC, Anderle P, Bürzle M, Suzuki Y, Freeman MR, Hediger MA, Kovacs G. Zinc transporters in prostate cancer. Mol Aspects Med. 2013;34(2-3):735-41. https://doi.org/10.1016/j.mam.2012.11.007
↑10. Fraum TJ, Ludwig DR, Kim EH, Schroeder P, Hope TA, Ippolito JE. Prostate cancer PET tracers: essentials for the urologist. Can J Urol. 2018;25(4):9371-9383. PMID: 30125515
↑11. Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202(3):654-62. https://doi.org/10.1002/jcp.20166
↑12. Chandler JD, Williams ED, Slavin JL, Best JD, Rogers S. Expression and localization of GLUT1 and GLUT12 in prostate carcinoma. Cancer. 2003;97(8):2035-42. https://doi.org/10.1002/cncr.11293
↑13. Reinicke K, Sotomayor P, Cisterna P, Delgado C, Nualart F, Godoy A. Cellular distribution of Glut-1 and Glut-5 in benign and malignant human prostate tissue. J Cell Biochem. 2012;113(2):553-62. https://doi.org/10.1002/jcb.23379
↑14. Xiao H, Wang J, Yan W, Cui Y, Chen Z, Gao X, Wen X, Chen J. GLUT1 regulates cell glycolysis and proliferation in prostate cancer. Prostate. 2018;78(2):86-94. https://doi.org/10.1002/pros.23448
↑15. Gonzalez-Menendez P, Hevia D, Alonso-Arias R, Alvarez-Artime A, Rodriguez-Garcia A, Kinet S, Gonzalez-Pola I, Taylor N, Mayo JC, Sainz RM. GLUT1 protects prostate cancer cells from glucose deprivation-induced oxidative stress. Redox Biol. 2018;17:112-127. https://doi.org/10.1016/j.redox.2018.03.017
↑16. Wang J, Xu W, Wang B, Lin G, Wei Y, Abudurexiti M, Zhu W, Liu C, Qin X, Dai B, Wan F, Zhang H, Zhu Y, Ye D. GLUT1 is an AR target contributing to tumor growth and glycolysis in castration-resistant and enzalutamide-resistant prostate cancers. Cancer Lett. 2020;485:45-55. https://doi.org/10.1016/j.canlet.2020.05.007
↑17. Garlapati C, Joshi S, Turaga RC, Mishra M, Reid MD, Kapoor S, Artinian L, Rehder V, Aneja R. Monoethanolamine-induced glucose deprivation promotes apoptosis through metabolic rewiring in prostate cancer. Theranostics. 2021;11(18):9089-9106. https://doi.org/10.7150/thno.62724
↑18. Cannistraci A, Hascoet P, Ali A, Mundra P, Clarke NW, Pavet V, Marais R. MiR-378a inhibits glucose metabolism by suppressing GLUT1 in prostate cancer. Oncogene. 2022;41(10):1445-1455. https://doi.org/10.1038/s41388-022-02178-0
↑19. Gasinska A, Jaszczynski J, Rychlik U, Łuczynska E, Pogodzinski M, Palaczynski M. Prognostic Significance of Serum PSA Level and Telomerase, VEGF and GLUT-1 Protein Expression for the Biochemical Recurrence in Prostate Cancer Patients after Radical Prostatectomy. Pathol Oncol Res. 2020;26(2):1049-1056. https://doi.org/10.1007/s12253-019-00659-4
↑20. Potter C, Harris AL. Hypoxia inducible carbonic anhydrase IX, marker of tumour hypoxia, survival pathway and therapy target. Cell Cycle. 2004;3(2):164-7. PMID: 14712082
↑21. Parkkila S. Significance of pH regulation and carbonic anhydrases in tumour progression and implications for diagnostic and therapeutic approaches. BJU Int. 2008;101 Suppl 4:16-21. https://doi.org/10.1111/j.1464-410X.2008.07643.x
↑22. Smyth LG, O'Hurley G, O'Grady A, Fitzpatrick JM, Kay E, Watson RW. Carbonic anhydrase IX expression in prostate cancer. Prostate Cancer Prostatic Dis. 2010;13(2):178-81. https://doi.org/10.1038/pcan.2009.58
↑23. Donato DP, Johnson MT, Yang XJ, Zynger DL. Expression of carbonic anhydrase IX in genitourinary and adrenal tumours. Histopathology. 2011;59(6):1229-39. https://doi.org/10.1111/j.1365-2559.2011.04074.x
↑24. Fiaschi T, Giannoni E, Taddei ML, Cirri P, Marini A, Pintus G, Nativi C, Richichi B, Scozzafava A, Carta F, Torre E, Supuran CT, Chiarugi P. Carbonic anhydrase IX from cancer-associated fibroblasts drives epithelial-mesenchymal transition in prostate carcinoma cells. Cell Cycle. 2013;12(11):1791-801. https://doi.org/10.4161/cc.24902
↑25. Ambrosio MR, Di Serio C, Danza G, Rocca BJ, Ginori A, Prudovsky I, Marchionni N, Del Vecchio MT, Tarantini F. Carbonic anhydrase IX is a marker of hypoxia and correlates with higher Gleason scores and ISUP grading in prostate cancer. Diagn Pathol. 2016;11(1):45. https://doi.org/10.1186/s13000-016-0495-1
↑26. Riemann A, Güttler A, Haupt V, Wichmann H, Reime S, Bache M, Vordermark D, Thews O. Inhibition of Carbonic Anhydrase IX by Ureidosulfonamide Inhibitor U104 Reduces Prostate Cancer Cell Growth, But Does Not Modulate Daunorubicin or Cisplatin Cytotoxicity. Oncol Res. 2018;26(2):191-200. https://doi.org/10.3727/096504017X14965111926391
For citations:
Vovdenko S.V., Morozov A.O., Avraamova S.T., Alexandrov N.S., Zharkov N.V., Kozlov V.V., Kogan E.A., Bezrukov E.A. The role of glucose transporter type 1 (GLUT1) and carbonic anhydrase IX (CAIX) expression by prostate adenocarcinoma tissue in determining disease prognosis and effectiveness of radical treatment. Urology Herald. 2022;10(4):13-20. (In Russ.) https://doi.org/10.21886/2308-6424-2022-10-4-13-20